Home> Modeling Basics > Modeling Kinetics > Reversable Reaction 1 Stella Tools
Reversible reactions are an important aspect of many biological processes. Let's look at reversible reaction models in Stella. We will use these models to discuss aspects of Stella and model development.
A generic example of a reversible reaction is the transformation of proteinA into proteinB and of proteinB into proteinA. A simple schematic drawn by hand, might be drawn like the following:
proteinA
![]() proteinB
proteinB
Our "hand" drawing tells us the structure of the reaction, what stocks (proteinA, proteinB) are involved and that ther is a flow of materials between the stocks. We assume that there is are rates of movement between these two states, even if we do not know what the rates are and there is no explicit indication of the rate in the drawing.
Instead of drawing a single arrow to represent both forward and backward reactions, we can draw two independent arrows to represent the two reactions.
proteinA ![]() proteinB
proteinB
In Stella, it would look like this:
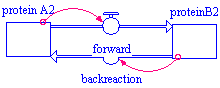
Again, we have two stocks of proteins (proteinA2 and proteinB2) set to some initial amount. The flow, reactionrates in Example1, is now drawn as two separate flows (forward and backreaction) to indicate that the materials go out from one stock and into the other. The connectors (red arrows) are drawn from the stocks to the flow whose reaction rate they help determine. (In Stella, the flows can be drawn as either uni- or bi-directional flows, as shown above.)
The structure in the hand drawn model and that in Stella are again similar.
Created by Dr. Raquell M. Holmes
Support for these pages was provided by EOT-PACI and NCSI, National Science Foundation (NSF) sponsored programs